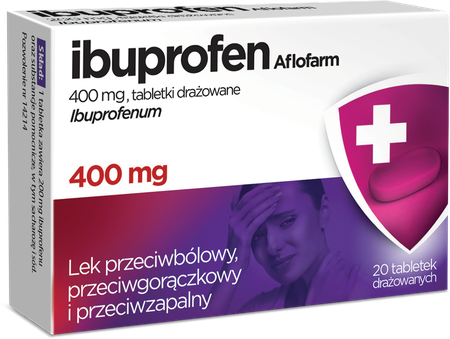
Ibuprofen Aflofarm 400 mg
Ibuprofen Aflofarm 400 mg are sugar-coated tablets indicated for use in cases of:
- pain of various etiologies of mild to moderate severity including headaches, migraines, toothaches, myalgia, lower back pains, bone and joint pains, and neuralgia;
- dysmenorrhea;
- fever (including flu, colds or other infectious diseases).
Pharmaceutical form diced tablets. 400 mg - 20 tab.
Category OTC
Additional information
Dosage and administration: Oral use.
Adults and children over 12 years of age: 1 tablet every 4 hours after a meal.
The tablets should be taken with water.
Do not take more than 3 tablets a day (maximum daily dose of 1200 mg of ibuprofen in divided doses).
The medicinal product should not be given to children under 12 years of age. Elderly: no dose adjustment is required.
Undesirable effects may be reduced by using the lowest effective dose for the shortest duration necessary to alleviate symptoms. If the medicinal product needs to be used for more than 3 days or the patient's condition worsens, contact a physician.
Active substance
One coated tablet contains 400 mg of ibuprofen (Ibuprofenum). Excipient with known effect: sucrose.
Indications
- pain of various etiologies of mild to moderate severity including headaches, migraines, toothaches, myalgia, lower back pains, bone and joint pains, and neuralgia;
- dysmenorrhea;
- fever (including flu, colds or other infectious diseases).
Contraindications
The medicinal product should not be used in patients:
- with hypersensitivity towards the active substance, any excipient and other non-steroidal anti-inflammatory drugs (NSAIDs);
- who have a history of allergy such as rhinitis, urticaria or bronchial asthma after taking acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (NSAIDs);
- with a history of stomach ulcer and/or active duodenal ulcer, or history of perforation or bleeding, also associated with the use of NSAIDs
- with severe hepatic insufficiency, severe renal insufficiency or severe heart failure (NYHA class IV);
- History of bronchial asthma, urticaria or allergic reaction after taking acetylsalicylic acid or other NSAIDs.
- taking other NSAIDs, including COX-2 inhibitors, at the same time (increased risk of side effects)
- during the third trimester of pregnancy
Marketing Authorisation Holder
Aflofarm Farmacja Polska Spółka z o.o.
Marketing Authorisation
14906
Information for the patient
Read the package leaflet for indications, contraindications, side effects, dosage as well as information on the use of this product, or consult your doctor or pharmacist before use. Misusing medicines may be dangerous to your life or health.
See how we care about quality
All our products are subject to strict safety requirements
We use rigorous quality control standards and procedures to ensure the highest level of safety for all our products.
See how we care about safety